1. About medis GmbH
medis Medizinische Messtechnik GmbH has been founded in 1991 as a spin-off of the Institute of Biomedical Engineering of the Technical University lmenau. The company is still based in the green heart of Germany in the Thuringian Forest in Ilmenau.

medis is a medium-sized company that is in no way inferior to its larger competitors. The highly qualified and experienced team specialises in the development and manufacture of innovative diagnostic devices, which are based on non-invasive examination technologies that enable the simultaneous analysis of different biomedical signals. This approach opens up new perspectives for extended patient monitoring and more comprehensive cardiovascular diagnostics. To meet customer needs, medis products are flexibly configurable and can be combined in various ways to fulfil a wide range of diagnostic requirements. medis is responsible for the international distribution of the complete product portfolio, with the aim of equipping hospitals, healthcare facilities and doctors.
2. Skills and facilities
With a qualified team, medis is able to carry out the development and production of medical measurement technology almost entirely in-house. A team of engineers and technicians works closely together to develop and manufacture innovative medical products according to documented processes. The product portfolio ranges from small, compact USB-powered devices to complex medical measurement systems with device carts and panel PCs.
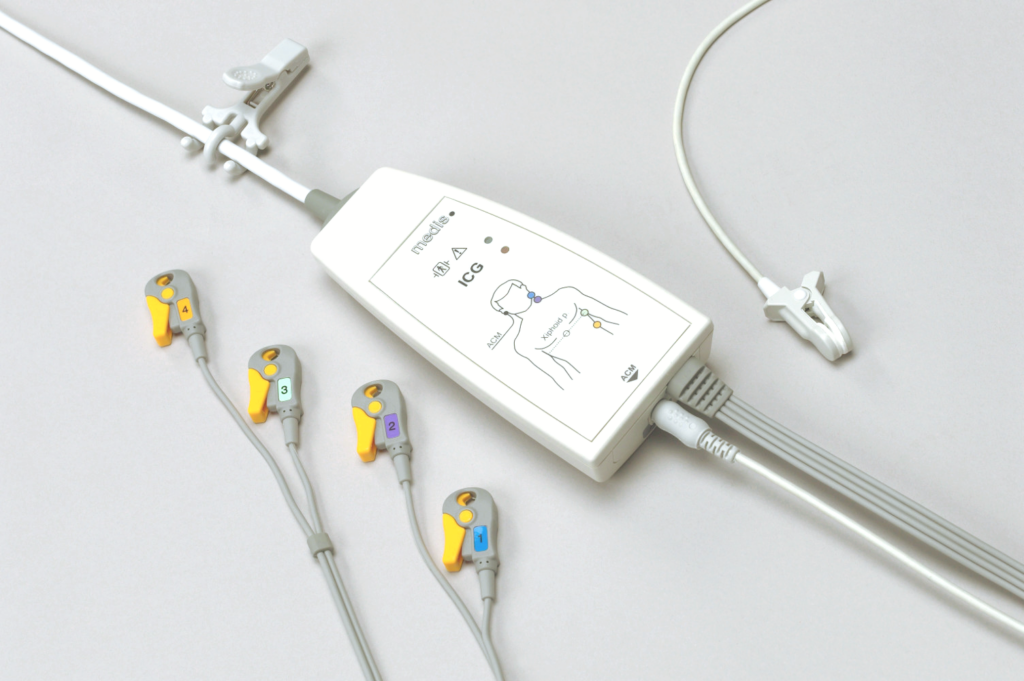
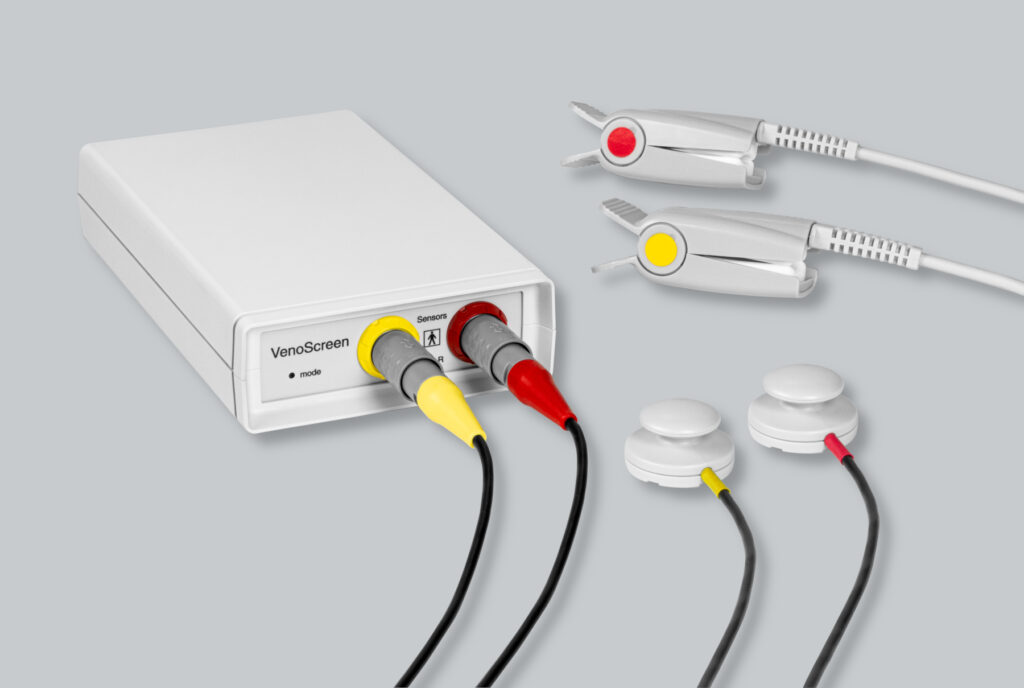
Regional suppliers are used to monitor and maintain high quality standards. Furthermore, technical employees carry out production and testing processes at the highest level, which is clearly reflected in the trend analysis in the Post-Market Surveillance. By carrying out 100% final testing in-house, we ensure high quality and thus stand behind our solid and long-term products.
In addition to product safety, it is also vital to consider permanent feedback from the market. By gathering feedback from customers and distributors, as well as conducting constant market observations, the company can incorporate the current requirements of the stakeholders into the existing products and processes. This helps to ensure a high standard in this area. In addition, the team is committed to developing and manufacturing products that meet the highest quality and safety standards, in accordance with the applicable law for medical devices. This commitment is confirmed annually through the maintenance of the EN ISO 13485 certificate.
3. Contribution to the ThrombUS+ project
medis supports the consortium of the ThrombUS+ project as an industry partner. The company has extensive expertise in impedance measurement technology and Light-Reflection-Rheography, and combines knowledge of these two methods being used in the project. The focus of the work packages was on Light-Reflection-Rheography (LRR), a special mode of Photo-Plethysmography that measures venous blood volume changes in the skin. medis is contributing its knowledge of the LRR method with the focus on using this procedure to diagnose deep vein thrombosis. A wireless version of the sensors is being developed and realised as a prototype, with the calculated parameters transmitted to the user interface via a wireless connection and processed there.
With many years of experience in a practical environment, the medis team can contribute a great deal of expertise regarding the development and placing on the market of medical devices. In addition, the team is well-positioned to offer insights from practical experiences, highlighting potential risks related to usability and real-life conditions in hospital settings. In comparison to the research institutions involved, the team possesses extensive knowledge of the application and implementation of regulatory requirements, which can be effectively transferred to project partners.




